The Science Behind Octolyte

Electrolyte additives that change what zinc batteries can do.
OctoLyte™ stops dendrite formation, reduces hydrogen, and maintains stability without degredation.
Inspired by electroplating in the electronics industry, our OctoLyte additives allow zinc battery recharging to take place with greater stability, significantly reduced dendrite formation, and low impedence.
Our Technology
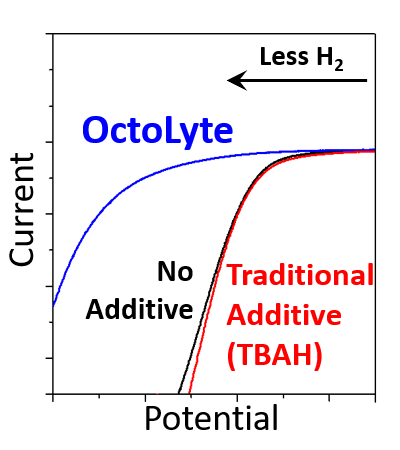
Reduced Hydrogen
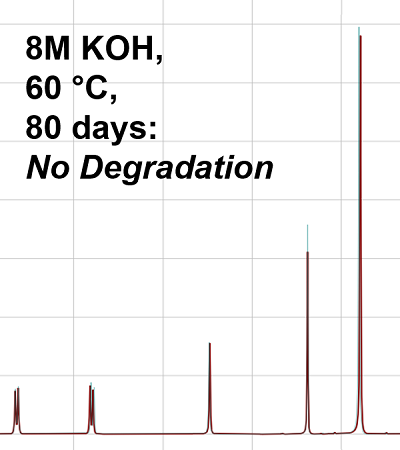
Long-Term Stability
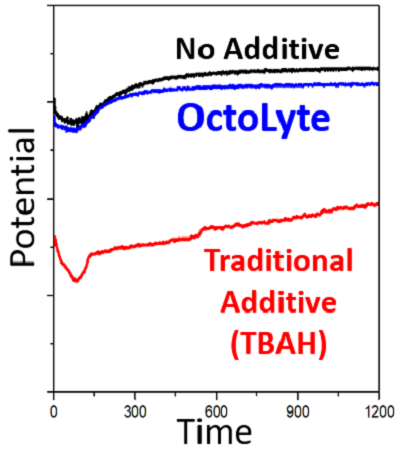
Low Polarization
How It Works
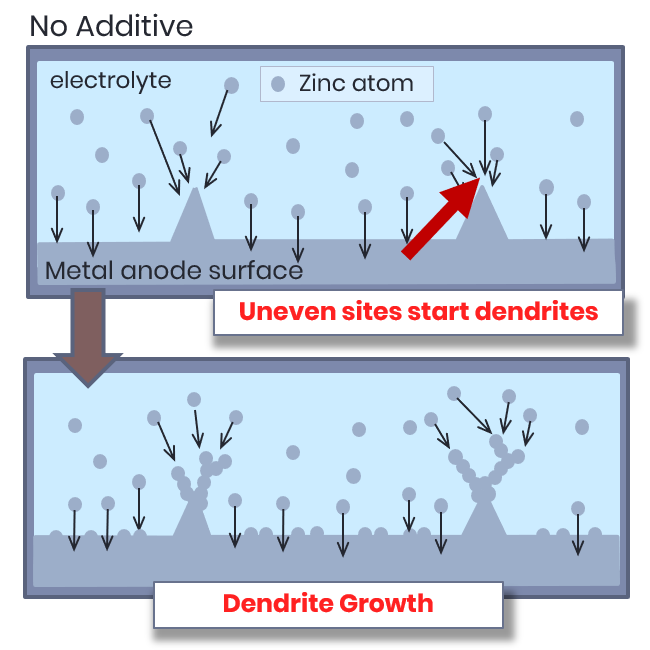
Typical Zinc Recharge
Uneven deposition leads to dendrites, unpredictable anode growth, lost capacity and lower cycle-life.
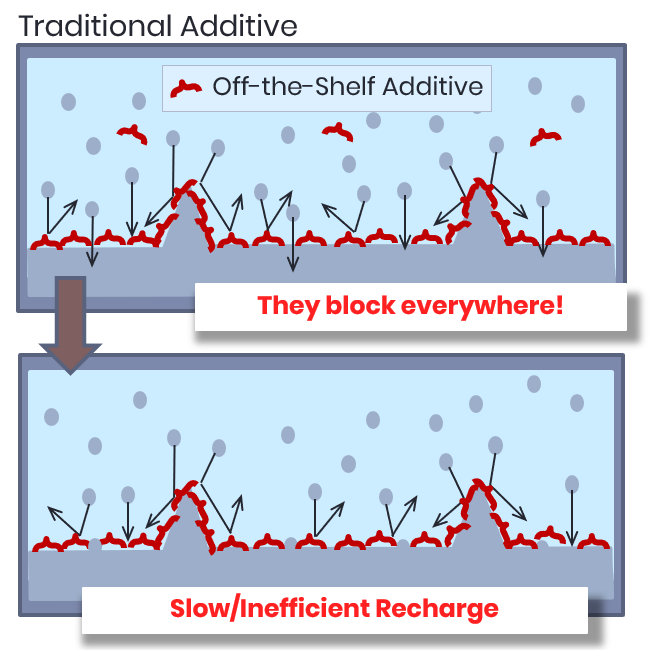
Traditional Additives
Unoptimized molecules block Zinc indiscriminately, preventing dendrites at a significant cost to efficiency.
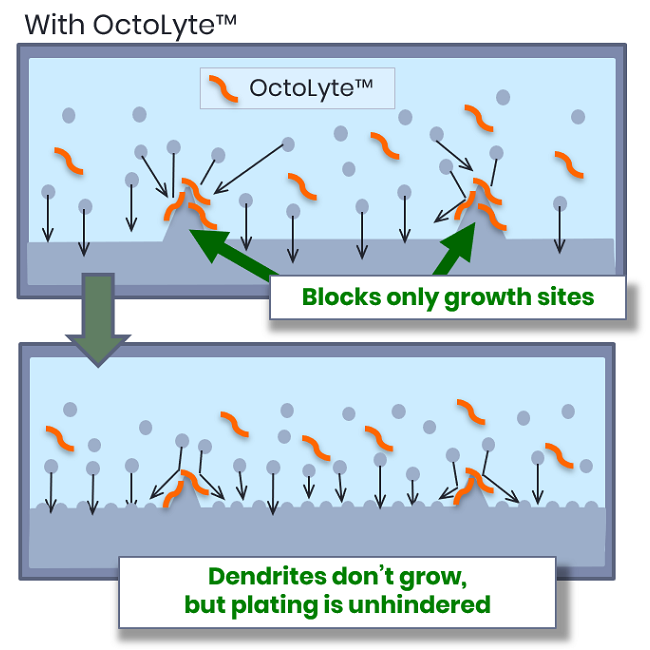
OctoLyte™’s Selective Control
Our designer additives affect only the uneven spots, stopping dendrites selectively without hurting efficiency.
